Griddle cakes include pancakes and crumpets. Pancakes are made from viscous and floury batters that retain some gas for the time they cook. The batter is poured onto a griddle and cooked until gas bubbles break the surface of the cake, then flipped to trap remaining gas. Crumpets are small flat, yeast-raised cakes with a pale, cratered surface. They are made from a thicker, bubbly pancake batter that is poured into ring molds. The cake is cooked slowly, unmolded, and turned over.
The fluffy texture of griddle pancakes depend on the development of tiny carbon dioxide gas pockets produced from the reaction of baking soda and an acid such as buttermilk. To obtain the best results, mix ingredients into a batter, then stop mixing before all the clumps of flour dissolve. Over-mixing causes flat, chewy pancakes due to the early escape of carbon dioxide and gluten overdevelopment. Allow the batter to sit for a while, preferably in the refridgerator. The cold temperature retards gluten development and decreases bacterial growth. With the right amount of gluten protein and CO2 gas, the batter becomes smooth. It spreads easily on the griddle, cooks evenly and thoroughly, and produces light-brown, fluffy pancakes.
Wednesday, February 29, 2012
Lesson 3: Buttermilk pancakes
This week the focus is on buttermilk pancakes. Topics include:
Griddle pancakes
Fresh fermented milks and creams
Maple and other syrups
Hope you join me!
Griddle pancakes
Fresh fermented milks and creams
Maple and other syrups
Hope you join me!
Saturday, February 18, 2012
Thoughts on Death by Chocolate
The road to eating a chocolate cookie is a long one. In this section I have included a significant number of posts related to history and processes for making chocolate, sugars, and vanilla. My intent is not to bore, but rather to provide insight on the complexities of food and hopefully encourage greater appreciation for the simple delight of eating a cookie.
1. There is so much to chocolate! Some of the take-home messages I got from learning about chocolate are:
3. High-fructose corn syrup has an ill reputation. Considering that fructose does not affect the body in the same way that sucrose does, the bad reputation seems undeserved. Moderation is key, but the world is too addicted to sweets. High-fructose corn syrup appears to be a decent attempt at satisfying a need, for a cheap price, without so many adverse effects.
There are too many thoughts to process. Feel free to comment.
1. There is so much to chocolate! Some of the take-home messages I got from learning about chocolate are:
- Eat more of it! High density chocolate low in sugar is good for my physical and mental health.
- Invest in high quality chocolate. Good chocolate can seem expensive, but given the process of making chocolate, it amazes me that I can buy a year-long process for just a few dollars.
- Along the same lines, it may also be worth it to invest in fair trade and conflict-free chocolate.
- Chocolate is for humans. Keep the furry paws (and mouths!) away from theobromine.
- Chocolate goes out of temper easily. Take care of chocolate, especially when travelling.
3. High-fructose corn syrup has an ill reputation. Considering that fructose does not affect the body in the same way that sucrose does, the bad reputation seems undeserved. Moderation is key, but the world is too addicted to sweets. High-fructose corn syrup appears to be a decent attempt at satisfying a need, for a cheap price, without so many adverse effects.
There are too many thoughts to process. Feel free to comment.
Death by Chocolate Cookies
Death By Chocolate Cookie
Adapted from a Baker’s Chocolate Ad, Feb. 2000.
Ingredients:
1 package chocolate squares (8 ounces, 8 squares)
8 oz. (1 cup) Chocolate pieces
3/4 cup firmly packed brown sugar
1/4 cup butter or margarine
2 eggs
1 tsp. vanilla
1 cup flour
1 tsp. baking powder
2 cups chopped nuts (optional)
Method:
Heat oven to 350 degrees F. Position the oven racks in the middle of the over or if there are multiple racks of cookies going in, put one rack in the bottom third and one rack in the top third of the oven.
Microwave 8 squares of chocolate in a large microwavable bowl with the butter on High for 1-2 minutes.
Stir until chocolate/butter mixture is melted and smooth
Stir in sugar, eggs and vanilla. Mix until smooth
Add the baking powder to the flour and mix. Then add the flour mixture to the chocolate mixture. Stir until smooth
Stir in chocolate pieces and nuts
Line cookie sheet with greased parchment paper.
Drop by tablespoon onto greased cookie sheet
Bake 8 minutes on bottom rack. Then rotate the rack and move to the top rack and cook for an additional 8 minutes. Cookies are done when they are puffed and feel set to the touch. It might require additional baking back on the bottom rack. You need to really watch the bottom baking to make sure you don’t burn your cookies
Cool on cookie sheet 1 minute.
Transfer to wire rack or parchment paper to cool completely.
Makes about 2 dozen cookies.
Notes:
If you are not a chocoholic and wish to reduce the amount of chocolate, you can add white chocolate chips or just eliminate the chocolate chips
Chemical leavenings
Chemical leavenings are a fast-acting source of gas. They exploit the reaction between acidic and alkaline compounds that result in carbon dioxide production.
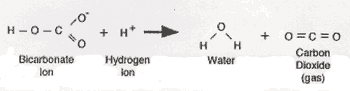
Baking soda, also known as sodium bicarbonate (NaHCO3), is the most common alkaline component in chemical leavenings. When mixed with an acid (H+), it reacts in the following way:
Fig 1:
Baking powders are complete leavening systems. They contain baking soda and an acid in the form of solid crystals. Ground dry starch is added to prevent premature reactions with moisture and add bulk to the mix. The timing of gas production varies according to the type of acid crystal used. If the acid is soluble (like cream of tartar), then the reactions occur quickly. If the acid is not very soluble, the reactions take longer. The acid remains in crystal form until the cooking temperature is high enough to dissolve it and promote the reaction with sodium bicarbonate.
"Double acting" baking powders inflate an initial set of gas bubbles upon mixing into the batter, and then a second set during the baking process. Baking powder for commercial purposes contains slow-release acids so that the leavening power is not lost during storage.
Chemical leaveners affect taste and color. Improper measuring and mixing can leave behind unreacted acids and bases that alter flavor. In slightly alkaline conditions, color changes occur as a result from enhanced browning reactions. Observable examples are chocolate turning red or blueberries turning green.
Baking soda, also known as sodium bicarbonate (NaHCO3), is the most common alkaline component in chemical leavenings. When mixed with an acid (H+), it reacts in the following way:
Fig 1:

If the dough or batter contains acids, only baking soda is necessary as a chemical leavening. Common acids in baking include: sourdough cultures, buttermilk, yogurt, brown sugar, molasses, chocolate, cocoa (not Dutch processed), fruit juices, and vinegar.
Baking powders are complete leavening systems. They contain baking soda and an acid in the form of solid crystals. Ground dry starch is added to prevent premature reactions with moisture and add bulk to the mix. The timing of gas production varies according to the type of acid crystal used. If the acid is soluble (like cream of tartar), then the reactions occur quickly. If the acid is not very soluble, the reactions take longer. The acid remains in crystal form until the cooking temperature is high enough to dissolve it and promote the reaction with sodium bicarbonate.
"Double acting" baking powders inflate an initial set of gas bubbles upon mixing into the batter, and then a second set during the baking process. Baking powder for commercial purposes contains slow-release acids so that the leavening power is not lost during storage.
Chemical leaveners affect taste and color. Improper measuring and mixing can leave behind unreacted acids and bases that alter flavor. In slightly alkaline conditions, color changes occur as a result from enhanced browning reactions. Observable examples are chocolate turning red or blueberries turning green.
Thursday, February 16, 2012
Sugars and Health
Empty calories
The concentration of calories in sugar is only second to fats and oils. The cautionary advise against consuming too much sugar derives from ingesting too many calories and displacing nutritious food with "empty calories.". Refined sugars are devoid of vitamins, minerals, and other nutritients that are important in a healthy diet.
Tooth decay
Certain kinds of Streptococcus colonize the mouth. The bacteria convert sugars into "plaque" carbohydrates, which anchor them to the teeth and form a protective layer. In addition, they also produce defensive acids that damage tooth enamel and lead to tooth decay.
(Interestingly, phenolic compounds such as are found in chocolate and tea interfere with the adhesion of bacteria to the teeth.)
Blood glucose
The body uses glucose as its primary source of energy. However, glucose is a highly reactive molecule. In excessive quantities, it can damage the eyes, kidneys, and both nervous and circulatory systems. The body controls glucose by removing it promptly from the blood via insulin, which helps distribute it to tissues for energy or storage. Intake of sugar overload stresses the insulin system. Diabetes may result from such strain.
Glycemic Index
Food varies in its content of sugars. The glycemic index measures how much a given food raises glucose levels. The foods that raise blood glucose level the most are ones rich in glucose such as rice, potatoes, and other starchy foods. Table sugar has a slightly lower glycemic index as it is a combination of glucose and fructose. Foods containing fructose have much lower glycemic indeces because fructose has to metabolize through the liver before it becomes available as a source of energy.
The concentration of calories in sugar is only second to fats and oils. The cautionary advise against consuming too much sugar derives from ingesting too many calories and displacing nutritious food with "empty calories.". Refined sugars are devoid of vitamins, minerals, and other nutritients that are important in a healthy diet.
Tooth decay
Certain kinds of Streptococcus colonize the mouth. The bacteria convert sugars into "plaque" carbohydrates, which anchor them to the teeth and form a protective layer. In addition, they also produce defensive acids that damage tooth enamel and lead to tooth decay.
(Interestingly, phenolic compounds such as are found in chocolate and tea interfere with the adhesion of bacteria to the teeth.)
Blood glucose
The body uses glucose as its primary source of energy. However, glucose is a highly reactive molecule. In excessive quantities, it can damage the eyes, kidneys, and both nervous and circulatory systems. The body controls glucose by removing it promptly from the blood via insulin, which helps distribute it to tissues for energy or storage. Intake of sugar overload stresses the insulin system. Diabetes may result from such strain.
Glycemic Index
Food varies in its content of sugars. The glycemic index measures how much a given food raises glucose levels. The foods that raise blood glucose level the most are ones rich in glucose such as rice, potatoes, and other starchy foods. Table sugar has a slightly lower glycemic index as it is a combination of glucose and fructose. Foods containing fructose have much lower glycemic indeces because fructose has to metabolize through the liver before it becomes available as a source of energy.
Sugar substitutes
Sugar alcohols, or polyols, provide bulk without being digested like sugars. Examples are sorbitol, mannitol, other sugars ending in -itol. They are derived from an altered sugar molecule which makes them hard to absorb and use. They only cause a slow rise in insulin levels. In addition, sugar alcohols lack the aldehyde group that initiates browning reactions, making them resistant to discoloration and flavor changes.
Intensive sweeteners can have a sweetness of 50 to 8000 times greater than table sugar. Examples include stevia, saccharin, and aspartame. Their advantage is that only small amounts are needed to attain sweetness, therefore the caloric content is negligible. The disadvantages are flavor differences with lingering after-tastes and heat instability.
Sweetness inhibitors, such as Lactisole, block the taste receptors for sweetness. They are used in roasted coffee, confectionery, and snacks. A tiny amount can reduce the apparent sweetness of sugar by two-thirds.
Intensive sweeteners can have a sweetness of 50 to 8000 times greater than table sugar. Examples include stevia, saccharin, and aspartame. Their advantage is that only small amounts are needed to attain sweetness, therefore the caloric content is negligible. The disadvantages are flavor differences with lingering after-tastes and heat instability.
Sweetness inhibitors, such as Lactisole, block the taste receptors for sweetness. They are used in roasted coffee, confectionery, and snacks. A tiny amount can reduce the apparent sweetness of sugar by two-thirds.
Kinds of sugar
White sugar is purified sucrose. It is obtained from sugar cane or beets that have been made into a juice, clarified, and converted into a dark syrup. The sucrose is later crystallized and centrifuged to remove impurities (molasses). Table sugar is around 99.85% pure sucrose. The differences in white sugars are attributed mostly to size and purity. Standard granulated table sugar has an approximate length of 0.3 to 0.5 mm.
Large grain sugars, such as coarse and sanding, measure 1 - 2 mm. These sugars are exceptionally pure batches of sucrose. They have been further washed with alcohol to remove impurities and sucrose dust to give them their characteristic sparkle and crystalline appearance.
Extra fine sugars like English caster sugars and baker's special are smaller than table sugar, measuring 0.1 to 0.3 mm. These sugars provide crystalline surfaces that can be used to introduce air into fat during the creaming stage of cake-making.
Powdered sugars offer no roughness to the tongue and measure 0.01 to 0.1 mm. These sugars contain 3% starch to absorb moisture and prevent caking.
Brown sugar is soft and clingy due to hygroscopic glucose and fructose molecules found in its molasses film. It contains a significant amount of water so keep in airtight container. At the same time, it traps air between groups of adhering crystals, so it is important to pack it down before its volume is measured. When exposed to air, brown sugar dries and becomes hard. To resoften, place a moistened cloth or an apple inside its container. The sugar absorbs the moisture from the cloth or fruit.
Brown sugars are divided into two subgroups:
Factory brown sugars are produced during the initial processing of the cane juice into unrefined sugar. These include demerara, turbinado and muscovado. These sugars retain a coating of the syrup from which they were crystalized.
Refinery brown sugars are produced at the refinery using raw sugar as the starting material, not the cane juice. Ordinary brown sugar is made this way. Some are dissolved and recrystalized, while others are made into white sugar and coated with a thin film of syrup or molasses.
Whole sugars are crystalline sugars still enveloped in the cooked cane juice. These sugars are usually found in international markets. Examples are jaggery (Indian) or piloncillo (Mexican/Latin America).
Molasses, also known as treacle (UK), is the syrup left over after the sugar cane sucrose has been removed. Most molasses today are blends of molasses and syrups obtained at various stages of sugar-making. The darker the molasses, the more its sugars have been caramelized by browning reactions. Therefore, the darker the molasses, the more bitter and less sweet. Their pH is unpredictable, usually between 5 and 7. It can sometimes react with baking soda and produce a leavening effect in baked goods. Molasses also help retain moisture and have antioxidant capacities. Unsulfured molasses refer to molasses that are dark in color and have not been treated with sulfur dioxide.
High-Fructose Corn Syrup is produced by adding enzymes to plain corn or potato syrups to convert some of the glucose sugars into fructose. The ratio then becomes 53% glucose and 42% fructose, with the same sweetness of table sugar. Its acidic pH (3.5 to 5.5) allows it to react with baking soda to produce carbon dioxide and act as a leavening tool. High-fructose corn syrup has long carbohydrate molecules that become tangled and result in a thicker consistency than any other sucrose syrup. This prevents crystallization and moisture loss. It also prolongs storage life.
Large grain sugars, such as coarse and sanding, measure 1 - 2 mm. These sugars are exceptionally pure batches of sucrose. They have been further washed with alcohol to remove impurities and sucrose dust to give them their characteristic sparkle and crystalline appearance.
Extra fine sugars like English caster sugars and baker's special are smaller than table sugar, measuring 0.1 to 0.3 mm. These sugars provide crystalline surfaces that can be used to introduce air into fat during the creaming stage of cake-making.
Powdered sugars offer no roughness to the tongue and measure 0.01 to 0.1 mm. These sugars contain 3% starch to absorb moisture and prevent caking.
Brown sugar is soft and clingy due to hygroscopic glucose and fructose molecules found in its molasses film. It contains a significant amount of water so keep in airtight container. At the same time, it traps air between groups of adhering crystals, so it is important to pack it down before its volume is measured. When exposed to air, brown sugar dries and becomes hard. To resoften, place a moistened cloth or an apple inside its container. The sugar absorbs the moisture from the cloth or fruit.
Brown sugars are divided into two subgroups:
Factory brown sugars are produced during the initial processing of the cane juice into unrefined sugar. These include demerara, turbinado and muscovado. These sugars retain a coating of the syrup from which they were crystalized.
Refinery brown sugars are produced at the refinery using raw sugar as the starting material, not the cane juice. Ordinary brown sugar is made this way. Some are dissolved and recrystalized, while others are made into white sugar and coated with a thin film of syrup or molasses.
Whole sugars are crystalline sugars still enveloped in the cooked cane juice. These sugars are usually found in international markets. Examples are jaggery (Indian) or piloncillo (Mexican/Latin America).
Molasses, also known as treacle (UK), is the syrup left over after the sugar cane sucrose has been removed. Most molasses today are blends of molasses and syrups obtained at various stages of sugar-making. The darker the molasses, the more its sugars have been caramelized by browning reactions. Therefore, the darker the molasses, the more bitter and less sweet. Their pH is unpredictable, usually between 5 and 7. It can sometimes react with baking soda and produce a leavening effect in baked goods. Molasses also help retain moisture and have antioxidant capacities. Unsulfured molasses refer to molasses that are dark in color and have not been treated with sulfur dioxide.
High-Fructose Corn Syrup is produced by adding enzymes to plain corn or potato syrups to convert some of the glucose sugars into fructose. The ratio then becomes 53% glucose and 42% fructose, with the same sweetness of table sugar. Its acidic pH (3.5 to 5.5) allows it to react with baking soda to produce carbon dioxide and act as a leavening tool. High-fructose corn syrup has long carbohydrate molecules that become tangled and result in a thicker consistency than any other sucrose syrup. This prevents crystallization and moisture loss. It also prolongs storage life.
Sunday, February 12, 2012
The molecules of sugar
Glucose, also known as dextrose, is the simplest sugar molecule. Starch is formed from chains of glucose molecules. It is most often encountered in the form of corn syrup, which is made by breaking starch down. Two glucose molecules form maltose. In comparison to sucrose, glucose is less sweet, less water-soluble, and thinner in solution. It caramelizes around 300 degrees F.
Fructose, also known as levulose, is an isomer of glucose. They have the same chemical formula but vary in their structure. Fructose is the sweetest of the common sugars and the most water-soluble. The human body metabolizes fructose more slowly than glucose and sucrose, making it a preferable sugar for diabetics. It caramelizes at 220 degrees F. Fructose molecules exist in various isomers that have different effects on our sweet receptors. The six-corner ring shape is the sweetest. It predominates in cold, acid solutions. This means that manufacturers can use half of the sugar content (and thus half of calories) by using fructose versus sucrose to reach the same sweetness in a cold drink. In warm conditions, the structure changes to a five-corner ring, which drops its sweetness to that of sucrose.
Sucrose, also known as table sugar. It is a compound made from one molecule of glucose and one molecule of fructose. Plants produce sucrose during photosynthesis. It is the form extracted from sugar cane and beets. It benefits from properties of glucose and fructose. It is the second sweetest, second most water-soluble, and most pleasant tasting even at high concentrations. It caramelizes at 340 degrees F. When heated in the presence of acid, sucrose breaks into its single molecules at a ratio of 75% glucose and fructose, and 25% sucrose. This process is referred to as inversion. Invert sugar exists only as a syrup since fructose cannot fully crystalize in the presence of glucose and sucrose. Invert sugars are useful in candy making.
Lactose is a composite of glucose and galactose. It is the sugar found in milk. It is much less sweet than sucrose. It is mostly used to add bulk than sweetness.
Fructose, also known as levulose, is an isomer of glucose. They have the same chemical formula but vary in their structure. Fructose is the sweetest of the common sugars and the most water-soluble. The human body metabolizes fructose more slowly than glucose and sucrose, making it a preferable sugar for diabetics. It caramelizes at 220 degrees F. Fructose molecules exist in various isomers that have different effects on our sweet receptors. The six-corner ring shape is the sweetest. It predominates in cold, acid solutions. This means that manufacturers can use half of the sugar content (and thus half of calories) by using fructose versus sucrose to reach the same sweetness in a cold drink. In warm conditions, the structure changes to a five-corner ring, which drops its sweetness to that of sucrose.
Sucrose, also known as table sugar. It is a compound made from one molecule of glucose and one molecule of fructose. Plants produce sucrose during photosynthesis. It is the form extracted from sugar cane and beets. It benefits from properties of glucose and fructose. It is the second sweetest, second most water-soluble, and most pleasant tasting even at high concentrations. It caramelizes at 340 degrees F. When heated in the presence of acid, sucrose breaks into its single molecules at a ratio of 75% glucose and fructose, and 25% sucrose. This process is referred to as inversion. Invert sugar exists only as a syrup since fructose cannot fully crystalize in the presence of glucose and sucrose. Invert sugars are useful in candy making.
Lactose is a composite of glucose and galactose. It is the sugar found in milk. It is much less sweet than sucrose. It is mostly used to add bulk than sweetness.
History of Sugar
Modern day, refined white sugar is a relative novelty. The sweet qualities of fruits and honey have been praised for nearly 4000 years, but refined sugar only became known to Europeans after 1100 BC. Even so, it remained a luxury until well into the 1700s.
Sugar cane has an unusually high sucrose content of 15%. Native to the New Guinea in the South Pacific, it was carried by prehistoric people into Asia. The sugar cane and the technology to make sugar traveled from Asia into the Middle East around the 6th century CE. Europeans were first exposed to sugar during the Crusades in the 11th century. Sugar was treated as a flavoring and a medicine. Medieval Europe used it to preserve fruits and flowers and as medicinal morsels used to mask bitter-tasting drugs. By the 14th century, sugar had already acquired popularity in non-medical confections. By the 16th century, confectionery had become an art. Cooks were already proficient in making syrups, hard candies, and other confections that were pleasing to see as well as to eat.
By the 18th century, the use of sugar in Europe exploded. Its consumption was supported by the colonial rule in the West Indies and the use of slavery. Columbus had carried the sugar cane to Hispaniola (now Haiti and the Dominican Republic) during his second voyage. By 1550, the Americas were producing sugar in significant quantities. By one estimate, two-thirds of the 20 million African slaves in America worked on sugar plantations. The intricate trade in sugar made major ports out of minor cities throughout Europe. The huge fortunes made by plantation owners funded much of the Industrial Revolution.
The 19th century brought a rapid decline to the sugar industry with the abolition of slavery and the development of an alternative to the sugar cane. In 1747, Andreas Marggraf, a Prussian chemist, discovered that using brandy to extract the juice of the white beet produced crystals identical to those from sugar cane. However, many years passed before the production of sugar from beets became appreciated. Emperor Napoleon awarded Benjamin Delessert for developing a working sugar-beet factory in 1812. Though a quick fad rose and faded, production of sugar from beets took a strong hold in the 1840s, and has been sustained ever since.
Today, 30% of sucrose production world wide comes from beets. Some sugar is still produced from sugar cane, but a majority of sugar comes from corn. Manufacturing sugar from corn has made sugar very inexpensive and abundant in the Western diet. Sugar production saw a 7-fold increase between 1900 and 1964; a growth unmatched by any other crop. Given the rising concerns of sugar in our diet today, there is an abundant market for products that mimic sugar without its adverse effects on body weight and health.
Sugar cane has an unusually high sucrose content of 15%. Native to the New Guinea in the South Pacific, it was carried by prehistoric people into Asia. The sugar cane and the technology to make sugar traveled from Asia into the Middle East around the 6th century CE. Europeans were first exposed to sugar during the Crusades in the 11th century. Sugar was treated as a flavoring and a medicine. Medieval Europe used it to preserve fruits and flowers and as medicinal morsels used to mask bitter-tasting drugs. By the 14th century, sugar had already acquired popularity in non-medical confections. By the 16th century, confectionery had become an art. Cooks were already proficient in making syrups, hard candies, and other confections that were pleasing to see as well as to eat.
By the 18th century, the use of sugar in Europe exploded. Its consumption was supported by the colonial rule in the West Indies and the use of slavery. Columbus had carried the sugar cane to Hispaniola (now Haiti and the Dominican Republic) during his second voyage. By 1550, the Americas were producing sugar in significant quantities. By one estimate, two-thirds of the 20 million African slaves in America worked on sugar plantations. The intricate trade in sugar made major ports out of minor cities throughout Europe. The huge fortunes made by plantation owners funded much of the Industrial Revolution.
The 19th century brought a rapid decline to the sugar industry with the abolition of slavery and the development of an alternative to the sugar cane. In 1747, Andreas Marggraf, a Prussian chemist, discovered that using brandy to extract the juice of the white beet produced crystals identical to those from sugar cane. However, many years passed before the production of sugar from beets became appreciated. Emperor Napoleon awarded Benjamin Delessert for developing a working sugar-beet factory in 1812. Though a quick fad rose and faded, production of sugar from beets took a strong hold in the 1840s, and has been sustained ever since.
Today, 30% of sucrose production world wide comes from beets. Some sugar is still produced from sugar cane, but a majority of sugar comes from corn. Manufacturing sugar from corn has made sugar very inexpensive and abundant in the Western diet. Sugar production saw a 7-fold increase between 1900 and 1964; a growth unmatched by any other crop. Given the rising concerns of sugar in our diet today, there is an abundant market for products that mimic sugar without its adverse effects on body weight and health.
Saturday, February 11, 2012
Vanilla
Vanilla, though one of the most widely used flavorings in the world, is only second to saffron in its cost of production. Most of the vanilla flavoring used today is synthetic.
Vanilla comes from the pod fruit of a climbing orchid native to Central and northern South America. Cultivated for more than a 1,000 years, the Aztecs used it as a flavoring for their chocolate. The Spaniards were introduced to it during colonization, and gave it its name. In the 19th century, Charles Morren, a Belgian botanist figured out how to pollinate vanilla flowers by hand. This discovery allowed for production of vanilla in areas that lacked the pollinating insects of Central America. The French took it to the islands of the coast of Africa, which now supply much of the world's Bourbon vanilla. Indonesia and Madagascar are today's largest vanilla producers.
The process for making vanilla is long and complex. Long after pollination, the vanilla orchids produce green pods that contain thousands of tiny seeds embedded in a mixture of sugars, fats, amino-acids, and phenolic-sugar storage compounds. The pods are exposed to high heat in order to prevent the pod from using up its sugars and amino acids, and to damage the pods' cells such that the phenolic storage compounds and browning enzymes (polyphenoloxidases) react. Phenolic compounds cluster into colored aggregates causing the vanilla pods to change color from green to brown. The pods are exposed to the sun for several days, then wrapped and allowed to "sweat." This process frees vanillin and related phenolic molecules from their sugar molecules, and develops the vanilla flavor. Finally, the pods are dried for several weeks, and aged to further develop their flavor. It takes 3-5 lbs of fresh pods to make 1 lb of cured beans.
Bourbon vanilla is considered to be the finest type, with the richest flavor. Indonesian beans are lighter and the pods contain less vanillin. Mexican beans contain about half the vanillin of Bourbon vanilla (though the process to develop the vanilla can last several months longer). Rare Tahitian vanilla beans have much less vanillin but carry unique flowery flavors.
Vanilla extracts are made by chopping vanilla beans, repeatedly exposing them to rinses of alcohol and water over the course of several days, and aging them to develop flavor. Artificial vanilla flavoring contains synthetic vanillin made from industrial by-products, such as wood lignin. Though the flavor is not complex like whole vanilla, the cost of production is about 100 times less than real vanilla.
The sticky, resinous material and tiny seeds of the vanilla bean can be easily scrapped and dispersed in food preparation directly. The fibrous pod wall can also be used to flavor dishes but must be soaked in either fat or alcohol to extract its flavor. Prepared vanilla extracts can be dispersed throughout a dish instantly. Keep in mind that they are best added toward the end of cooking as any time spent at high temperatures reduces their aroma.
Vanilla comes from the pod fruit of a climbing orchid native to Central and northern South America. Cultivated for more than a 1,000 years, the Aztecs used it as a flavoring for their chocolate. The Spaniards were introduced to it during colonization, and gave it its name. In the 19th century, Charles Morren, a Belgian botanist figured out how to pollinate vanilla flowers by hand. This discovery allowed for production of vanilla in areas that lacked the pollinating insects of Central America. The French took it to the islands of the coast of Africa, which now supply much of the world's Bourbon vanilla. Indonesia and Madagascar are today's largest vanilla producers.
The process for making vanilla is long and complex. Long after pollination, the vanilla orchids produce green pods that contain thousands of tiny seeds embedded in a mixture of sugars, fats, amino-acids, and phenolic-sugar storage compounds. The pods are exposed to high heat in order to prevent the pod from using up its sugars and amino acids, and to damage the pods' cells such that the phenolic storage compounds and browning enzymes (polyphenoloxidases) react. Phenolic compounds cluster into colored aggregates causing the vanilla pods to change color from green to brown. The pods are exposed to the sun for several days, then wrapped and allowed to "sweat." This process frees vanillin and related phenolic molecules from their sugar molecules, and develops the vanilla flavor. Finally, the pods are dried for several weeks, and aged to further develop their flavor. It takes 3-5 lbs of fresh pods to make 1 lb of cured beans.
Bourbon vanilla is considered to be the finest type, with the richest flavor. Indonesian beans are lighter and the pods contain less vanillin. Mexican beans contain about half the vanillin of Bourbon vanilla (though the process to develop the vanilla can last several months longer). Rare Tahitian vanilla beans have much less vanillin but carry unique flowery flavors.
Vanilla extracts are made by chopping vanilla beans, repeatedly exposing them to rinses of alcohol and water over the course of several days, and aging them to develop flavor. Artificial vanilla flavoring contains synthetic vanillin made from industrial by-products, such as wood lignin. Though the flavor is not complex like whole vanilla, the cost of production is about 100 times less than real vanilla.
The sticky, resinous material and tiny seeds of the vanilla bean can be easily scrapped and dispersed in food preparation directly. The fibrous pod wall can also be used to flavor dishes but must be soaked in either fat or alcohol to extract its flavor. Prepared vanilla extracts can be dispersed throughout a dish instantly. Keep in mind that they are best added toward the end of cooking as any time spent at high temperatures reduces their aroma.
Chocolate in research
Aside from benefits already discussed, research provides us with even more reasons to consume chocolate.
Japanese researchers (Matsui, 2005) have concluded that consuming cocoa can prevent obesity in high-fat diets. Their research shows that cocoa suppresses the expression of genes that code for enzymes involved in the synthesis of fatty acids in the liver and white adipose tissues. Cocoa ingestion also decreases gene expression for fatty-acid transport molecules. In addition, cocoa enhances the mechanism by which more energy is obtained from fat tissues. As a result, rats who were fed a high-fat diet but supplemented with real cocoa had significantly lower body weights, less adipose tissue, and lower serum triglycerol concentrations than rats who were on the same high fat diet but did not supplement with cocoa. (1)
Furthermore, Swiss scientists (Martin, 2009) conducted a research that suggests that daily consumption of 40 g of dark chocolate during a 2-week period is sufficient to decrease levels of stress hormones. (2)
Sources to the research mentioned above:
1. - Matsui, N., et al. "Ingested Cocoa can Prevent High-fat Diet-induced Obesity by Regulating the Expression of Genes for Fatty Acid Metabolism." Nutrition 21 (2005): 594-601.
2. - Martin, F.P., et al. "Metabolic effects of dark chocolate consumption on energy, gut microbiota, and stress-related metabolism in free-living subjects." J Proteome Res. 2009 Dec;8(12):5568-79.
Japanese researchers (Matsui, 2005) have concluded that consuming cocoa can prevent obesity in high-fat diets. Their research shows that cocoa suppresses the expression of genes that code for enzymes involved in the synthesis of fatty acids in the liver and white adipose tissues. Cocoa ingestion also decreases gene expression for fatty-acid transport molecules. In addition, cocoa enhances the mechanism by which more energy is obtained from fat tissues. As a result, rats who were fed a high-fat diet but supplemented with real cocoa had significantly lower body weights, less adipose tissue, and lower serum triglycerol concentrations than rats who were on the same high fat diet but did not supplement with cocoa. (1)
Furthermore, Swiss scientists (Martin, 2009) conducted a research that suggests that daily consumption of 40 g of dark chocolate during a 2-week period is sufficient to decrease levels of stress hormones. (2)
Sources to the research mentioned above:
1. - Matsui, N., et al. "Ingested Cocoa can Prevent High-fat Diet-induced Obesity by Regulating the Expression of Genes for Fatty Acid Metabolism." Nutrition 21 (2005): 594-601.
2. - Martin, F.P., et al. "Metabolic effects of dark chocolate consumption on energy, gut microbiota, and stress-related metabolism in free-living subjects." J Proteome Res. 2009 Dec;8(12):5568-79.
Friday, February 10, 2012
Chocolate and Health
Cocoa beans are like other seeds in that they contain high amounts of saturated fats, used in the plant to support the embryo until it sprouts roots and leaves. However, the fat content can be compared to that of avocados or olive oil. Much of the fat in cocoa butter is stearic acid, which is quickly converted into oleic acid, an unsaturated fatty acid.
Chocolate consumption is believed to reduce the risk of heart disease in the way that red wine flavanols do. Cocoa particles are a rich source of antioxidant phenolic compounds, which prevent fat-like substances such as LDL (Low Density Lipoproteins) from oxidizing and clogging arteries. Added sugar or milk dilutes the cocoa solids and their phenolics. The dutching process also reduces phenolics in cocoa. Milk proteins bind phenolics and prevent us from absorbing them. Therefore, the type and quality of chocolate consumed matters in relation to its effects on heart health.
There is no link established for the consumption of chocolate and acne. Chocolate is not a causative agent for acne, nor does it have any effect on acne. It does not worsen a condition or make it better. Acne is more likely to be affected by stress level and hormones than diet.
Chocolate is not responsible for cavities. The high-sugar content in some chocolate is more likely to be the culprit than the cocoa parts. In fact, cocoa butter has been used to coat teeth and prevent plaque formation.
Eating chocolate may have some psychological benefits as discussed in the section Chocolate and the Brain.
Chocolate consumption is believed to reduce the risk of heart disease in the way that red wine flavanols do. Cocoa particles are a rich source of antioxidant phenolic compounds, which prevent fat-like substances such as LDL (Low Density Lipoproteins) from oxidizing and clogging arteries. Added sugar or milk dilutes the cocoa solids and their phenolics. The dutching process also reduces phenolics in cocoa. Milk proteins bind phenolics and prevent us from absorbing them. Therefore, the type and quality of chocolate consumed matters in relation to its effects on heart health.
There is no link established for the consumption of chocolate and acne. Chocolate is not a causative agent for acne, nor does it have any effect on acne. It does not worsen a condition or make it better. Acne is more likely to be affected by stress level and hormones than diet.
Chocolate is not responsible for cavities. The high-sugar content in some chocolate is more likely to be the culprit than the cocoa parts. In fact, cocoa butter has been used to coat teeth and prevent plaque formation.
Eating chocolate may have some psychological benefits as discussed in the section Chocolate and the Brain.
Chocolate toxicity in dogs - theobromine
Theobromine is the compound in chocolate responsible for chocolate toxicity in dogs. Dogs metabolize theobromine very slowly in comparison to humans. The half life of theobromine is 17.5 hrs in dogs compared to 3 hrs in humans. Lethal toxicity in dogs is noted as 100-150 mg of theobromine per kg of body weight in dogs, though its toxic effects can be observed at much lower concentrations.
There are variables to take into account when dealing with chocolate/theobromine toxicity.
The weight of the dog.
Smaller dogs are many times more susceptible to toxicity. The ratio of mg/kg is much higher. Dogs are gorgers. Small dogs may have less body mass and weight, but can consume high amounts of chocolate if readily available.
The theobromine content in the chocolate eaten
The same amount of baker's chocolate is potentially more lethal than milk chocolate due to the content of cocoa solids. The more diluted the cocoa content in a chocolate product, the lesser the threat. This does not mean that it is ok to give dogs small amounts of chocolate. Even small amounts can lead to toxicity, even if it is not lethal. Dogs will suffer vomiting and diarreah after eating small amounts of chocolate as their body attempts to prevent further theobromine absorbtion. White chocolate is not dangerous since it does not contain theobromine.
Beware of cocoa shell mulches used in landscaping. Cocoa shells naturally contain theobromine. Manufacturers often strip theobromine from the mulch, but not always. Check for content if your pet likes to eat the mulch.
So what happens when a dog consumes chocolate?
Theobromine stimulates the Central Nervous System (CNS). The dog shows signs of hyperactivity and restlessness, increased urine output, and irregular heartbeat. According to the Merck Veterinary Manual, Methylxanthines like theobromine increase intracellular calcium levels that lead to increased strength and contractility of skeletal and cardiac muscle. They compete for receptors in the CNS and inhibit esterases that result in increased cyclic AMP levels. They also increase circulating levels of epinephrine and norepinephrine. In severe cases, these chemical changes cause dogs to have tremors and seizures, hypertension, increased body temperature, and difficulty breathing. Coma and death may also result.
Other species are also susceptible to theobromine including cats and rabbits. However, other species are less prone to consume chocolate. Seek veterinary care immediately if you suspect your pet has ingested chocolate.
There are variables to take into account when dealing with chocolate/theobromine toxicity.
The weight of the dog.
Smaller dogs are many times more susceptible to toxicity. The ratio of mg/kg is much higher. Dogs are gorgers. Small dogs may have less body mass and weight, but can consume high amounts of chocolate if readily available.
The theobromine content in the chocolate eaten
The same amount of baker's chocolate is potentially more lethal than milk chocolate due to the content of cocoa solids. The more diluted the cocoa content in a chocolate product, the lesser the threat. This does not mean that it is ok to give dogs small amounts of chocolate. Even small amounts can lead to toxicity, even if it is not lethal. Dogs will suffer vomiting and diarreah after eating small amounts of chocolate as their body attempts to prevent further theobromine absorbtion. White chocolate is not dangerous since it does not contain theobromine.
Beware of cocoa shell mulches used in landscaping. Cocoa shells naturally contain theobromine. Manufacturers often strip theobromine from the mulch, but not always. Check for content if your pet likes to eat the mulch.
So what happens when a dog consumes chocolate?
Theobromine stimulates the Central Nervous System (CNS). The dog shows signs of hyperactivity and restlessness, increased urine output, and irregular heartbeat. According to the Merck Veterinary Manual, Methylxanthines like theobromine increase intracellular calcium levels that lead to increased strength and contractility of skeletal and cardiac muscle. They compete for receptors in the CNS and inhibit esterases that result in increased cyclic AMP levels. They also increase circulating levels of epinephrine and norepinephrine. In severe cases, these chemical changes cause dogs to have tremors and seizures, hypertension, increased body temperature, and difficulty breathing. Coma and death may also result.
Other species are also susceptible to theobromine including cats and rabbits. However, other species are less prone to consume chocolate. Seek veterinary care immediately if you suspect your pet has ingested chocolate.
Chocolate and the Brain
Eating chocolate has long been associated with a feeling of well-being, alertness, and satisfaction. Researchers have identified some key components in chocolate that account for the emotions of eating chocolate.
Anandamide
Anandamide is a naturally-occurring neurotransmitter molecule synthesized in areas of the human brain important in enforcing and breaking short term neural connections. Its role is important in memory, higher thought processes, movement control, and forgetfulness. Anandamide binds to the so-called "bliss" receptor. Its effects include dulling pain and inducing an overall feeling of well-being. Unfortunately for humans, anandamide is a fragile molecule which breaks down easily.
Researchers have isolated at least three compounds in chocolate that strongly resemble anandamide. Those compounds are able to bind to the bliss receptor and produce effects similar to those of anandamide. Furthermore, they have also found in chocolate numerous other compounds that inhibit anandamide breakdown. According to the research of Daniele Piomelli and Emmanuelle diTomaso, chocolate has "pharmalogically-active substances that...may be responsible for certain drug-induced psychoses associated with chocolate craving."
Theobromine
According to xocoatl.org, the often-quoted caffeine content of chocolate is a myth. The stimulant effects often attributed to caffeine, are in fact, the effects of theobromine, which makes up about 1-2% of cocoa by weight. Like caffeine, theobromine increases the activity of neurotransmitters responsible for increased attention and alertness. But unlike caffeine, theobromine is a much milder stimulant to the central nervous system and is not physiologically addictive. Theobromine also increases a sense of well-being as a mild anti-depressant. It is proven to be a cause of physical and mental relaxation. It stimulates the cardiovascular and muscular systems. All in all, theobromine may be responsible for giving chocolate its aphrodisiac charactersitics.
Though chocolate contains trace amounts of cannabinoid and phenylethylamine chemicals, research has shown that there are no chemical properties that would cause a physical dependency. Craving chocolate and satisfying the crave has more to do with the sensory experience of consuming chocolate than meeting a physiological need.
The following websites are good sources for more information on chocolate and its compounds:
http://antoine.frostburg.edu/chem/senese/101/features/anandamide.shtml
http://www.xocoatl.org/
Anandamide
Anandamide is a naturally-occurring neurotransmitter molecule synthesized in areas of the human brain important in enforcing and breaking short term neural connections. Its role is important in memory, higher thought processes, movement control, and forgetfulness. Anandamide binds to the so-called "bliss" receptor. Its effects include dulling pain and inducing an overall feeling of well-being. Unfortunately for humans, anandamide is a fragile molecule which breaks down easily.
Researchers have isolated at least three compounds in chocolate that strongly resemble anandamide. Those compounds are able to bind to the bliss receptor and produce effects similar to those of anandamide. Furthermore, they have also found in chocolate numerous other compounds that inhibit anandamide breakdown. According to the research of Daniele Piomelli and Emmanuelle diTomaso, chocolate has "pharmalogically-active substances that...may be responsible for certain drug-induced psychoses associated with chocolate craving."
Theobromine
According to xocoatl.org, the often-quoted caffeine content of chocolate is a myth. The stimulant effects often attributed to caffeine, are in fact, the effects of theobromine, which makes up about 1-2% of cocoa by weight. Like caffeine, theobromine increases the activity of neurotransmitters responsible for increased attention and alertness. But unlike caffeine, theobromine is a much milder stimulant to the central nervous system and is not physiologically addictive. Theobromine also increases a sense of well-being as a mild anti-depressant. It is proven to be a cause of physical and mental relaxation. It stimulates the cardiovascular and muscular systems. All in all, theobromine may be responsible for giving chocolate its aphrodisiac charactersitics.
Though chocolate contains trace amounts of cannabinoid and phenylethylamine chemicals, research has shown that there are no chemical properties that would cause a physical dependency. Craving chocolate and satisfying the crave has more to do with the sensory experience of consuming chocolate than meeting a physiological need.
The following websites are good sources for more information on chocolate and its compounds:
http://antoine.frostburg.edu/chem/senese/101/features/anandamide.shtml
http://www.xocoatl.org/
Tips on working with chocolate
Chocolate is easy to incorporate, but there are a couple things to keep in mind:
1. Chocolate is extremely dry and it does not mix well with moisture. Even a little bit of water added to molten chocolate can be disastrous. When water comes into contact with sugar and cocoa particles, it creates a type of syrup that makes the particles stick together and precipitate out from the liquid cocoa butter. Therefore, add solid chocolate to hot liquid ingredients, or pour liquid all at once into the molten chocolate, not gradually. If water does come into contact with molten chocolate, try adding more warm water to turn the paste into a thick fluid instead.
2. Chocolates are not interchangeable. Chocolate varies in proportions of cocoa butter, cocoa solids, milk solids, sugars, etc. When following/writing recipes, it is important to note what chocolate type is needed. Recipes may rely on the sugar content of the chocolate for the syrup qualities formed, which in turn affect the fluidity of the recipe. If a higher percentage cocoa is used instead of a sweet cocoa, then that cocoa will absorb moisture, reduce fluid volume, and reduce fluidity. Likewise, it is important to note whether the cocoa powder needed is alkalized or not. Some recipes may rely on acidic natural cocoa to react with baking soda to generate carbon dioxide for rising. If the cocoa used is alkalized, such reaction does not occur.
1. Chocolate is extremely dry and it does not mix well with moisture. Even a little bit of water added to molten chocolate can be disastrous. When water comes into contact with sugar and cocoa particles, it creates a type of syrup that makes the particles stick together and precipitate out from the liquid cocoa butter. Therefore, add solid chocolate to hot liquid ingredients, or pour liquid all at once into the molten chocolate, not gradually. If water does come into contact with molten chocolate, try adding more warm water to turn the paste into a thick fluid instead.
2. Chocolates are not interchangeable. Chocolate varies in proportions of cocoa butter, cocoa solids, milk solids, sugars, etc. When following/writing recipes, it is important to note what chocolate type is needed. Recipes may rely on the sugar content of the chocolate for the syrup qualities formed, which in turn affect the fluidity of the recipe. If a higher percentage cocoa is used instead of a sweet cocoa, then that cocoa will absorb moisture, reduce fluid volume, and reduce fluidity. Likewise, it is important to note whether the cocoa powder needed is alkalized or not. Some recipes may rely on acidic natural cocoa to react with baking soda to generate carbon dioxide for rising. If the cocoa used is alkalized, such reaction does not occur.
Thursday, February 9, 2012
Kinds of chocolate
Unsweetened chocolate contains only cocoa solids and cocoa butter. It is the closest to natural chocolate. Bitter, baking, and cooking chocolate all refer to unsweetened chocolate.
Sweet chocolate is the type that is consumed the most. It includes any chocolate that has any amount of sugar added to it. It can further be broken down:
White chocolate contains no cocoa particles, only deodorized cocoa butter, milk solids, and sugar. It has no chocolate flavor.
Cocoa powder is formed from cocoa bean particles whose cocoa butter has been extracted. Cocoa powder is the most concentrated form of chocolate. It has a strong chocolate taste, pronounced bitterness, and acidity. Its pH is around 5.
"Dutched" or alkanized cocoa is cocoa powder that has been treated with potassium carbonate to raise its pH to 7 or 8. The alkaline treatement reduces the level of flavorful molecules such as pyrazines, thiazoles, and furaneol because they bond to each other to form flavorless pigments. Thus, the darker the Dutched cocoa, the milder its flavor. Bakers should beware when recipes call for cocoa powder vs. Dutch process cocoa powder as some recipes rely on either the acidity or alkalinity for baking reactions.
Instant cocoa includes lecithin, an emulsifier that makes the cocoa more water soluble. The sugar content of instant cocoa can make up to 70% of its weight. It contains minimum amounts of cocoa solids.
Sweet chocolate is the type that is consumed the most. It includes any chocolate that has any amount of sugar added to it. It can further be broken down:
- Dark chocolate does not contain milk solids. The contents of cocoa solids, cocoa butter, and sugar depend on the chocolate variety: bitter, bittersweet, or sweet. For example, 62% chocolate contains 62% cocoa butter and cocoa solids by weight, and 38% sugar and other ingredients such as lecithin and vanilla. Finer chocolates have a higher cocoa liquor content, usually betwen 50% and 72%. Strong chocolates have a stronger flavor, and are excellent in cooking/baking. (note: German chocolate is a type of dark chocolate, sweeter than bittersweet, but not as sweet as sweet chocolate. It refers to a specific brand of chocolate developed in the 1950s.)
- Milk chocolate is the most popular variety. It is the mildest flavor as it contains a large portion of sugar and milk solids. The minimum amount of chocolate liquor required is 10%, but good quality chocolate has upwards of 40%. Milk chocolate has a minimum of 12% whole milk. This chocolate tends to be softer.
White chocolate contains no cocoa particles, only deodorized cocoa butter, milk solids, and sugar. It has no chocolate flavor.
Cocoa powder is formed from cocoa bean particles whose cocoa butter has been extracted. Cocoa powder is the most concentrated form of chocolate. It has a strong chocolate taste, pronounced bitterness, and acidity. Its pH is around 5.
"Dutched" or alkanized cocoa is cocoa powder that has been treated with potassium carbonate to raise its pH to 7 or 8. The alkaline treatement reduces the level of flavorful molecules such as pyrazines, thiazoles, and furaneol because they bond to each other to form flavorless pigments. Thus, the darker the Dutched cocoa, the milder its flavor. Bakers should beware when recipes call for cocoa powder vs. Dutch process cocoa powder as some recipes rely on either the acidity or alkalinity for baking reactions.
Instant cocoa includes lecithin, an emulsifier that makes the cocoa more water soluble. The sugar content of instant cocoa can make up to 70% of its weight. It contains minimum amounts of cocoa solids.
Unique qualities of chocolate
Chocolate is a remarkable food. No other food is as versatile in terms of consistency, flavor, and shape.
The unique consistency of chocolate derives from the physical qualities of cocoa butter. The structure of cacao fat molecules is mostly saturated and unusually regular, containing just three kinds of fatty acids. Thus the fat molecules are able to form a compact network of stable crystals with a little liquid fat to move between the cystals. This structure only results if the chocolate has been carefully tempered. Chocolate that melts and resolidifies in an uncontrolled way develops an unstable structure. The network is less organized; it has a higher content of liquid fat, and uneven crystals form. The resulting chocolate is soft, greasy, and mottled.
The richness and complexity of chocolate is unique among foods. Chemists have identified over 600 volatile molecules in chocolate. Both, its intrinsic flavor and complex preparation give chocolate its flavor depth.
Few other foods can be molded in as many ways as chocolate can. It is hard and dry at room temperature, but melts just below human body temperature. Since the process involves a liquid state that is then cooled and solidified, chocolate can be shaped into almost any shape.
The unique consistency of chocolate derives from the physical qualities of cocoa butter. The structure of cacao fat molecules is mostly saturated and unusually regular, containing just three kinds of fatty acids. Thus the fat molecules are able to form a compact network of stable crystals with a little liquid fat to move between the cystals. This structure only results if the chocolate has been carefully tempered. Chocolate that melts and resolidifies in an uncontrolled way develops an unstable structure. The network is less organized; it has a higher content of liquid fat, and uneven crystals form. The resulting chocolate is soft, greasy, and mottled.
The richness and complexity of chocolate is unique among foods. Chemists have identified over 600 volatile molecules in chocolate. Both, its intrinsic flavor and complex preparation give chocolate its flavor depth.
Few other foods can be molded in as many ways as chocolate can. It is hard and dry at room temperature, but melts just below human body temperature. Since the process involves a liquid state that is then cooled and solidified, chocolate can be shaped into almost any shape.
Chocolate Production
There are three groups of cacao trees: the Criollos, Forasteros, and Trinitarios. The Criollos produce some of the finest flavors, but are disease prone and low-yielding trees. They account for less than 5% of the world crop. The Forasteros provide full-flavored beans and are high-yielding. They account for most of the world crop. Trinitarios are hybrids of the other two.
On plantations and farms, the cacao pods are opened and their contents exposed. The sweet pulp is fermented for 2-8 days. Fermentation of the pulp is a key step in making chocolate flavorful. Three phases occur during fermentation: First, yeasts convert sugars to alcohols and metabolize some of the acids in the pulp. Next, as the oxygen supply in the pods diminishes, lactic acid bacteria attack the pods. Some of these lactic acid bacteria are the same species found in fermented dairy. Last, acetic acid bacteria consume the alcohol produced by the yeast and convert it into acetic acid. The acetic acid then penetrates into the beans, making the cacao beans less astringent. Digestive enzymes within the beans break down proteins and sucrose, which will later produce more aromatic molecules during roasting. The beans soak in some of the flavor of the fermented pulp, which makes the beans more flavorful.
The beans are dried to about 7% moisture, at which point they are resistant to further microbial spoilage. The beans are cleaned and shipped to manufacturers.
The next step is roasting. Manufacturers roast beans to develop their flavor. The roasting needs of cacao beans are milder than those of coffee beans because cacao has an abundance of reactive amino acids that participate in Maillard browning to generate flavor. Therefore, roasting helps preserve the rich flavors within the beans acquired during fermentation.
The shells and nibs are separated after roasting. The nibs are ground into cocoa liquor. After that, the process varies according to the ultimate product desired. For cocoa powder, the cocoa liquor is pressed to remove the cocoa butter, then pulverized. For chocolate, other ingredients are added to the liquor (sugar, milk, vanilla, etc.) and then subjected to conching-a process of extended agitation and added heat. The physical friction breaks up particles of the other ingredients so that they coat the cocoa butter evenly. It also mellows the strong flavor of cocoa by means of aeration. Volatile compounds present in the cocoa evaporate, including acids and aldehydes. Favorable volatiles, such as pyrazines, furaneol, and maltol, become concentrated. These compounds make up much of the characteristic aromas in chocolate. At the end of conching, cocoa butter and lecithin are added to create the creamy texture of chocolate.
Following conching, the liquid chocolate needs to be tempered. Tempering is a process that involves heating and cooling the liquid chocolate to ensure that cocoa butter crystals stabilize and become uniform in size.
Lastly, chococolate is molded and cooled off.
On plantations and farms, the cacao pods are opened and their contents exposed. The sweet pulp is fermented for 2-8 days. Fermentation of the pulp is a key step in making chocolate flavorful. Three phases occur during fermentation: First, yeasts convert sugars to alcohols and metabolize some of the acids in the pulp. Next, as the oxygen supply in the pods diminishes, lactic acid bacteria attack the pods. Some of these lactic acid bacteria are the same species found in fermented dairy. Last, acetic acid bacteria consume the alcohol produced by the yeast and convert it into acetic acid. The acetic acid then penetrates into the beans, making the cacao beans less astringent. Digestive enzymes within the beans break down proteins and sucrose, which will later produce more aromatic molecules during roasting. The beans soak in some of the flavor of the fermented pulp, which makes the beans more flavorful.
The beans are dried to about 7% moisture, at which point they are resistant to further microbial spoilage. The beans are cleaned and shipped to manufacturers.
The next step is roasting. Manufacturers roast beans to develop their flavor. The roasting needs of cacao beans are milder than those of coffee beans because cacao has an abundance of reactive amino acids that participate in Maillard browning to generate flavor. Therefore, roasting helps preserve the rich flavors within the beans acquired during fermentation.
The shells and nibs are separated after roasting. The nibs are ground into cocoa liquor. After that, the process varies according to the ultimate product desired. For cocoa powder, the cocoa liquor is pressed to remove the cocoa butter, then pulverized. For chocolate, other ingredients are added to the liquor (sugar, milk, vanilla, etc.) and then subjected to conching-a process of extended agitation and added heat. The physical friction breaks up particles of the other ingredients so that they coat the cocoa butter evenly. It also mellows the strong flavor of cocoa by means of aeration. Volatile compounds present in the cocoa evaporate, including acids and aldehydes. Favorable volatiles, such as pyrazines, furaneol, and maltol, become concentrated. These compounds make up much of the characteristic aromas in chocolate. At the end of conching, cocoa butter and lecithin are added to create the creamy texture of chocolate.
Following conching, the liquid chocolate needs to be tempered. Tempering is a process that involves heating and cooling the liquid chocolate to ensure that cocoa butter crystals stabilize and become uniform in size.
Lastly, chococolate is molded and cooled off.
History of Chocolate
History
Chocolate comes from the cacao plant, originally evolved in South America. The cacao tree bears large seed pods that contain a white, sweet pulp. Embedded in the pulp are the dark-colored cacao seeds. It is believed that the Olmecs of the southern Gulf coast of Mexico were the first to cultivate the cacao trees. Before 600 BCE, they introduced it to the Maya in the Yucatan peninsula. The Maya then traded it to the Aztecs in the north. The Aztecs roasted and grounded the cacao seeds and made them into a bitter drink that was associated with their religious ceremonies. The seeds were valuable enough that were used as currency.
Europeans first saw the cacao bean around 1502. There are detailed descriptions of cacao and its usage in the New World dating in the early 1500s. According to the History of the New World (1564) by Girolamo Benzoni, the cacao kernels were laid to dry, then roasted and ground, finally mixed with water and other spices (including chili pepper) for a drink that was satisfying without intoxicating. The Aztecs highly esteemed their chocolate drink. According to the English Jesuit Thomas Gage, they also dried the cocoa beans and spices, ground them, heated them to melt and form a paste. Then they allowed the paste to solidify on a leaf, and peeled it off as a chocolate tablet, which they later mixed with atole to make hot chocolate.
By the 1580s, the first chocolate factories were built in Spain. Within 70 years, chocolate was found in Italy, France, and England. The Europeans substituted chili pepper with sugar. By the late 17th century, chocolate houses had begun to serve hot chocolate with milk. By this time, the Spanish had also made chocolate into lozenges, and people were aware that taking the chocolate at night would keep them up, so it was good for soldiers. By the 18th century, chocolate had made its way into many recipes throughout Europe.
Still, chocolate was mainly thought of as a drink, not a solid food. The chocolate paste was coarse and crumbly in texture. It was not until 1828, when Conrad van Houten in Amsterdam developed a hydrolic press that removed more than half of the cocoa butter from the ground bean. He then pulverized the defatted cacao beans to become "cocoa." He treated the cocoa with alkaline salts to make it more water soluble. The result was what we now know as Dutch processed cocoa. Later, he found that the cocoa butter could be added to a paste of ordinary ground chocolate and sugar, and the resulting paste had a smoother texture that allowed for the first solid chocolate. The English firm of Fry and Sons in 1847 introduced the first chocolate that could be eaten, rather than made into a drink.
In 1876, Swiss Daniel Peter used dry milk powder produced by Henri Nestlé to make the first solid milk chocolate. Most chocolate today is consumed as milk chocolate. Two years later, in 1878, Rudolphe Lindt invented the conche, a machine that ground cacao beans, sugar, and milk powder to a much finer consitency, which is the consistency of chocolate consumed today.
The process for making chocolate has not changed that much since the early discoveries in the 19th century.Today, over half of the world cacao is produced in West Africa. Other major producers are Indonesia and Brazil.
Chocolate comes from the cacao plant, originally evolved in South America. The cacao tree bears large seed pods that contain a white, sweet pulp. Embedded in the pulp are the dark-colored cacao seeds. It is believed that the Olmecs of the southern Gulf coast of Mexico were the first to cultivate the cacao trees. Before 600 BCE, they introduced it to the Maya in the Yucatan peninsula. The Maya then traded it to the Aztecs in the north. The Aztecs roasted and grounded the cacao seeds and made them into a bitter drink that was associated with their religious ceremonies. The seeds were valuable enough that were used as currency.
Europeans first saw the cacao bean around 1502. There are detailed descriptions of cacao and its usage in the New World dating in the early 1500s. According to the History of the New World (1564) by Girolamo Benzoni, the cacao kernels were laid to dry, then roasted and ground, finally mixed with water and other spices (including chili pepper) for a drink that was satisfying without intoxicating. The Aztecs highly esteemed their chocolate drink. According to the English Jesuit Thomas Gage, they also dried the cocoa beans and spices, ground them, heated them to melt and form a paste. Then they allowed the paste to solidify on a leaf, and peeled it off as a chocolate tablet, which they later mixed with atole to make hot chocolate.
By the 1580s, the first chocolate factories were built in Spain. Within 70 years, chocolate was found in Italy, France, and England. The Europeans substituted chili pepper with sugar. By the late 17th century, chocolate houses had begun to serve hot chocolate with milk. By this time, the Spanish had also made chocolate into lozenges, and people were aware that taking the chocolate at night would keep them up, so it was good for soldiers. By the 18th century, chocolate had made its way into many recipes throughout Europe.
Still, chocolate was mainly thought of as a drink, not a solid food. The chocolate paste was coarse and crumbly in texture. It was not until 1828, when Conrad van Houten in Amsterdam developed a hydrolic press that removed more than half of the cocoa butter from the ground bean. He then pulverized the defatted cacao beans to become "cocoa." He treated the cocoa with alkaline salts to make it more water soluble. The result was what we now know as Dutch processed cocoa. Later, he found that the cocoa butter could be added to a paste of ordinary ground chocolate and sugar, and the resulting paste had a smoother texture that allowed for the first solid chocolate. The English firm of Fry and Sons in 1847 introduced the first chocolate that could be eaten, rather than made into a drink.
In 1876, Swiss Daniel Peter used dry milk powder produced by Henri Nestlé to make the first solid milk chocolate. Most chocolate today is consumed as milk chocolate. Two years later, in 1878, Rudolphe Lindt invented the conche, a machine that ground cacao beans, sugar, and milk powder to a much finer consitency, which is the consistency of chocolate consumed today.
The process for making chocolate has not changed that much since the early discoveries in the 19th century.Today, over half of the world cacao is produced in West Africa. Other major producers are Indonesia and Brazil.
Lesson 2: Death by Chocolate
This week we will explore one of my favorite food items: chocolate! The hands-on assignment is chocolate cookies, so the notes will include the following topics:
All things chocolate
Chocolate in research
Vanilla
Sugars
Baking powder & Baking soda
Hope you join me!
All things chocolate
Chocolate in research
Vanilla
Sugars
Baking powder & Baking soda
Hope you join me!
Sunday, February 5, 2012
Thoughts on guacamole and salsa
I hope the notes below relating to ingredients of guacamole and salsa have been informative. Here are a few points/questions to consider:
1. Would the addition of chilies to guacamole prevent browning due to their high ascorbic acid content?
2. Browning or Maillard Reactions seem to be prevalent in nature. It is notable that organisms have developed defense mechanisms at all levels of evolution. I will continue to explore this topic in further detail as it pertains to other food.
3. The idea that spicy food affects our brains via the "pain" pathway is interesting to me. Are spicy foods proof that humans can experience 'pleasurable pain'?
4. I enjoyed thinking of fruits and vegetables as live organisms. It interests me to consider the breakdown of fruit in relation to processes of aging and dying. Enzymatic processes become random and chemical reactions upset the cells' balance leading to its death.
5. The effects of water crystallization on vegetables extends farther than that of simply freezing and thawing the water molecules. Uncontrolled water crystals actually puncture cell walls, thus attributing the limp texture of thawed vegetables. The physical structure is damaged and as a result the inner contents of the cells spill. When thawing frozen fruits and vegetables, one is losing more than just water content.
I'll limit my inquiries to five per session. Feel free to post your own thoughts.
1. Would the addition of chilies to guacamole prevent browning due to their high ascorbic acid content?
2. Browning or Maillard Reactions seem to be prevalent in nature. It is notable that organisms have developed defense mechanisms at all levels of evolution. I will continue to explore this topic in further detail as it pertains to other food.
3. The idea that spicy food affects our brains via the "pain" pathway is interesting to me. Are spicy foods proof that humans can experience 'pleasurable pain'?
4. I enjoyed thinking of fruits and vegetables as live organisms. It interests me to consider the breakdown of fruit in relation to processes of aging and dying. Enzymatic processes become random and chemical reactions upset the cells' balance leading to its death.
5. The effects of water crystallization on vegetables extends farther than that of simply freezing and thawing the water molecules. Uncontrolled water crystals actually puncture cell walls, thus attributing the limp texture of thawed vegetables. The physical structure is damaged and as a result the inner contents of the cells spill. When thawing frozen fruits and vegetables, one is losing more than just water content.
I'll limit my inquiries to five per session. Feel free to post your own thoughts.
Friday, February 3, 2012
Chilies
Chilies are the most widely grown spice in the world. Capsaicin is the active ingredient that gives chilies their "spicyness." In terms of evolution, the chili peppers developed capsaicin as a chemical repellant against mammals, whose teeth grind up the fruit and destroy the seeds. Birds, on the other hand, are immune to capsaicin. They swallow the fruits whole and disperse the seeds widely.
Capsaicin is synthesized by the surface cells of the placenta - the pithy tissue that bears the seeds. It accumulates in droplets just under the cuticle of the placenta surface, not on the seeds, but it can coat the seeds quickly if that cuticle is placed under pressure. Some capsaicin enters the body of the fruit via the plant's circulation.
Capsaicin pungency is strongest before ripening. The hottest chilies are the ones picked around the time they start to change colors. There are different versions of the capsaicin molecule which account for the difference in pungency - quick and transient or slow and persistent.
The effects of capsaicin on the body are many. It affects the body's temperature regulation. It makes us feel hot thus activating our cooling mechanism of sweating and increased blood flow to the skin. Capsaicin increases metabolic rate and triggers brain signals to make us feel less hungry and more satisfied. (Weight loss, anyone?) Capsaicin is a skin and eye irritant. Its oily nature makes it difficult to wash off surfaces, so care must be taken when handling chilies.
To quench the burn, try something either cold (ice) or solid and rough (rice, crackers, a spoonful of sugar). Cold liquid cools the receptors below the tempertature at which they are activated. Cold water does not help as much because capsaicin is not water-soluble. Water distributes more capsaicin particles to the mouth. Cold milk is more successful. Carbonation in drinks adds to the irritation, alcohol has no effect. Rough solid food distracts the nerves with a different signal to the brain.
How do we rate chili peppers? The Scoville test for Capsaicinoids. Capsaicin is measured in parts per million and converted into Scoville heat units: 1 ppm = 15 Scoville heat units. The hottest peppers are the habaneros (200K - 300K Scoville Units), whereas the mildest are bell peppers (0 Scoville Units).
Research on chili peppers is ongoing. Healthwise, there is no evidence that chili peppers lead to ulcers. Instead, research sugggests that chilis may have health benefits. Capsaicin has been found to have anticoagulant properties. Chilis are high in vitamin C, as much or more than citrus fruits. Some research is focused on the understanding pain pathways (and pain-killers) via the capsaicin receptor in the brain, which is in the "pain" pathway.
Capsaicin is synthesized by the surface cells of the placenta - the pithy tissue that bears the seeds. It accumulates in droplets just under the cuticle of the placenta surface, not on the seeds, but it can coat the seeds quickly if that cuticle is placed under pressure. Some capsaicin enters the body of the fruit via the plant's circulation.
Capsaicin pungency is strongest before ripening. The hottest chilies are the ones picked around the time they start to change colors. There are different versions of the capsaicin molecule which account for the difference in pungency - quick and transient or slow and persistent.
The effects of capsaicin on the body are many. It affects the body's temperature regulation. It makes us feel hot thus activating our cooling mechanism of sweating and increased blood flow to the skin. Capsaicin increases metabolic rate and triggers brain signals to make us feel less hungry and more satisfied. (Weight loss, anyone?) Capsaicin is a skin and eye irritant. Its oily nature makes it difficult to wash off surfaces, so care must be taken when handling chilies.
To quench the burn, try something either cold (ice) or solid and rough (rice, crackers, a spoonful of sugar). Cold liquid cools the receptors below the tempertature at which they are activated. Cold water does not help as much because capsaicin is not water-soluble. Water distributes more capsaicin particles to the mouth. Cold milk is more successful. Carbonation in drinks adds to the irritation, alcohol has no effect. Rough solid food distracts the nerves with a different signal to the brain.
How do we rate chili peppers? The Scoville test for Capsaicinoids. Capsaicin is measured in parts per million and converted into Scoville heat units: 1 ppm = 15 Scoville heat units. The hottest peppers are the habaneros (200K - 300K Scoville Units), whereas the mildest are bell peppers (0 Scoville Units).
Research on chili peppers is ongoing. Healthwise, there is no evidence that chili peppers lead to ulcers. Instead, research sugggests that chilis may have health benefits. Capsaicin has been found to have anticoagulant properties. Chilis are high in vitamin C, as much or more than citrus fruits. Some research is focused on the understanding pain pathways (and pain-killers) via the capsaicin receptor in the brain, which is in the "pain" pathway.
Ripening
Ripening is the fruits last intense phase of life before starting to decay. Ethylene is the single trigger for the ripening enzymes to go into action.
There are two styles of ripening among fruits:
1. Climacteric - the fruit stimulates itself by producing more ethylene and begins to respire (O2 consumption and CO2 production) much faster than before. These are fruits that are harvested mature but green, and will ripen on their own. Examples are: bananas, avocados, pears, and tomatoes. (Note: climacteric fruit will still taste better if allowed to ripen on the plant).
2. Nonclimacteric - fruits that don't respond to ethylene in a positive feedback loop. They ripen gradually, don't store sugars as starch, and depend on their connection to the parent plant for ripening. Examples are: pineapples, citrus fruits, most berries, and melons. These are best when picked as ripe as possible.
There are two styles of ripening among fruits:
1. Climacteric - the fruit stimulates itself by producing more ethylene and begins to respire (O2 consumption and CO2 production) much faster than before. These are fruits that are harvested mature but green, and will ripen on their own. Examples are: bananas, avocados, pears, and tomatoes. (Note: climacteric fruit will still taste better if allowed to ripen on the plant).
2. Nonclimacteric - fruits that don't respond to ethylene in a positive feedback loop. They ripen gradually, don't store sugars as starch, and depend on their connection to the parent plant for ripening. Examples are: pineapples, citrus fruits, most berries, and melons. These are best when picked as ripe as possible.
Thursday, February 2, 2012
Enzymatic Browning
Ever wonder what happens when a fruit or vegetable discolors soon after being cut? Here is the answer from On Food and Cooking, The Science and Lore of the Kitchen, p. 269:
"[The] discoloration is caused by three chemical ingredients: 1- and 2-ring phenolic compounds, certain plant enzymes, and oxygen. In the intact fruit or vegetable, the phenolic compounds are kept in the storage vacuole, the enzymes in the surrounding cytoplasm. When the cell structure is damaged and phenolics are mixed with enzymes and oxygen, the enzymes oxidize the phenolics, forming molecules that eventually react with each other and bond together into light-absorbing clusters."
It is a similar principle to when people react to sun exposure and become tan.
So why does lemon juice prevent browning?
Browning enzymes work very slowly in acidic conditions. Aside from its acidity, lemon juice also has ascorbic acid (Vitamin C) which has antioxidant properties.
"[The] discoloration is caused by three chemical ingredients: 1- and 2-ring phenolic compounds, certain plant enzymes, and oxygen. In the intact fruit or vegetable, the phenolic compounds are kept in the storage vacuole, the enzymes in the surrounding cytoplasm. When the cell structure is damaged and phenolics are mixed with enzymes and oxygen, the enzymes oxidize the phenolics, forming molecules that eventually react with each other and bond together into light-absorbing clusters."
It is a similar principle to when people react to sun exposure and become tan.
So why does lemon juice prevent browning?
Browning enzymes work very slowly in acidic conditions. Aside from its acidity, lemon juice also has ascorbic acid (Vitamin C) which has antioxidant properties.
Avocado
Most avocados in the US are grown in Southern California. The most common variety is the Haas avocado, which belongs to the Guatemalan group, which means they are not very cold-tolerant and can suffer injury in the fridge.
Avocados don't ripen until after they are picked. To ripen an avocado, keep at room temperature in a paper bag with either an apple or a banana. These fruits emit ethylene, which aids the ripening process. Do not place an avocado in the fridge while unripe. It will not ripen. Once ripen, the avocado can be stored in the fridge for several days.
To prevent the avocado from browning, add an acidic ingredient such as lemon juice, or keep in airtight wrapping.
Avocados don't ripen until after they are picked. To ripen an avocado, keep at room temperature in a paper bag with either an apple or a banana. These fruits emit ethylene, which aids the ripening process. Do not place an avocado in the fridge while unripe. It will not ripen. Once ripen, the avocado can be stored in the fridge for several days.
To prevent the avocado from browning, add an acidic ingredient such as lemon juice, or keep in airtight wrapping.
Alliums
The Allium genus includes: onions, garlic, and leeks.
The strong, often pungent, flavor of the onion comes from its defensive use of sulfur.
The alliums stores sulfur as chemical ammunition, which float in the cell fluids. An enzyme trigger is held separately in a storage vacuole. When a raw onion, garlic, or leek is chopped, chewed, or otherwise physically damaged, the enzyme trigger is released from its vacuole, breaks the ammunition molecules in half to produce sulfurous molecules, which are strong-smelling and irritating. Some of these molecules are volatile, so they continue to react into other compounds. Exposure to oxygen will affect the reactions and thus the flavor of the raw allium. Chopping, pureeing, and pounding will produce varying results.
If the allium is to be consumed raw, it's best to rinse it first to remove the sulfur compounds, since they will continue to react and become harsher with time and exposure to air.
Lacrimator is the sulfur compound that causes the eyes to water. This compound escapes the onion and goes into the eyes and nose of the onion cutter, where it attacks nerve endings directly. Its effects can be minimized by chilling the onions in ice water 30-60 min.
The method of cooking will also affect how the sulfur compounds react, thus producing a range of characteristic flavors.
Cooking at high temperatures in fat will give the strongest flavors. Blanching garlic inactivates the flavor-generating enzyme, so it gives a milder flavor.
The strong, often pungent, flavor of the onion comes from its defensive use of sulfur.
The alliums stores sulfur as chemical ammunition, which float in the cell fluids. An enzyme trigger is held separately in a storage vacuole. When a raw onion, garlic, or leek is chopped, chewed, or otherwise physically damaged, the enzyme trigger is released from its vacuole, breaks the ammunition molecules in half to produce sulfurous molecules, which are strong-smelling and irritating. Some of these molecules are volatile, so they continue to react into other compounds. Exposure to oxygen will affect the reactions and thus the flavor of the raw allium. Chopping, pureeing, and pounding will produce varying results.
If the allium is to be consumed raw, it's best to rinse it first to remove the sulfur compounds, since they will continue to react and become harsher with time and exposure to air.
Lacrimator is the sulfur compound that causes the eyes to water. This compound escapes the onion and goes into the eyes and nose of the onion cutter, where it attacks nerve endings directly. Its effects can be minimized by chilling the onions in ice water 30-60 min.
The method of cooking will also affect how the sulfur compounds react, thus producing a range of characteristic flavors.
Cooking at high temperatures in fat will give the strongest flavors. Blanching garlic inactivates the flavor-generating enzyme, so it gives a milder flavor.
Handling and storing fruits and vegetables
First, let's talk a bit about handling and storing fruits and vegetables.
The deterioration of a harvested vegetables can be noted within a few hours of picking. This is due to the plants' cells effort to stay alive. Plant cells consume themselves and accumulate waste products, thus affecting their taste and texture. Corn and peas use their sugars for energy, celery uses its water to retain turgid pressure. Give them long enough and they lose their flavor and crispiness as they begin to die.
In contrast, fruits may get better after harvesting because they continue to ripen. But past ripening, they too run out of energy and die.
Spoilage is further brought on by microbes. Vegetables are mainly attacked by bacteria (Erwinia, Pseudomonas - "soft rot"). Fruits resist bacteria due to their acidity but are attacked by yeasts and molds (Penicillin, Botrytis).
For best results, it is important to know how to choose, handle, and store produce.
Produce with high metabolism will spoil faster: mushrooms, berries, apricots, figs, avocados, and papayas.
Lethargic produce such as apples, pears, kiwi, cabbage, and carrots are good keepers.
Moldy fruit or vegetables should be discarded immediately, as it can lead to "an infection"and spoil the rest of the produce faster. Clean out fridge drawers and fruit bowls often to reduce the microbial population.
It's best to keep produce in restricted spaces within the fridge to slow down moisture loss, but since produce exhales carbon dioxide and water, it's important to also avoid too much condensation. (Now I get why the fridge drawers are labeled "high/low moisture!") Tip: Line containers with paper towel.
Restricted spaces will also limit access to too much oxygen, which increases metabolic activity, but not so much that the cells go into anaerobic metabolism, which generates alcohols (think fermentation) and leads to tissue damage.
It's a tricky balance because keeping produce in a closed bag will create a favorable environment of higher CO2 and low O2, but it will also trap ethylene, a plant hormone that advances ripening and accelerates aging. Permanganate inserts destroy ethylene, so inserts may extend storage life.
Cooling extends the life of produce by: 1. slowing metabolism, 2. slowing microbial growth. But beware of refrigerating fruits and vegetables that are native to warmer regions. The cold leads to cell malfunction, uncontrolled enzyme action, and tissue injury. Foods to better keep at room temperature include: melons, eggplants, squash, tomatoes, cucumbers, peppers, beans, bananas, avocados, and citrus fruits.
Freezing kills plant tissue in two ways: 1. as the water crystallizes, enzymes and other reactive molecules become concentrated and react abnormally. 2. water crystals puncture cell walls and membranes, causing a physical disruption. Flash freezing at extreme low temperatures creates smaller crystals, thus lessening the damage.
Blanching is a technique where the food is rapidly immersed in boiling water for a minute or two, then rapidly immersed in cold water, and then frozen. The boiling will inactivate enzymes, the cold water will stop the food from continuing to cook and softening the cell walls.
Sugar syrup can prevent enzymatic browning in fruit (when accompanied by ascorbic acid) and improve the texture of the frozen fruit by stiffening the cell walls when absorbed.
Frozen produce should be wrapped in air- and watertight containers to minimize freezer burn (which is caused by sublimation of water molecules).
Phew! So many Do's and Don'ts just to keep good tasting (and nutritious) fruits and vegetables.
The deterioration of a harvested vegetables can be noted within a few hours of picking. This is due to the plants' cells effort to stay alive. Plant cells consume themselves and accumulate waste products, thus affecting their taste and texture. Corn and peas use their sugars for energy, celery uses its water to retain turgid pressure. Give them long enough and they lose their flavor and crispiness as they begin to die.
In contrast, fruits may get better after harvesting because they continue to ripen. But past ripening, they too run out of energy and die.
Spoilage is further brought on by microbes. Vegetables are mainly attacked by bacteria (Erwinia, Pseudomonas - "soft rot"). Fruits resist bacteria due to their acidity but are attacked by yeasts and molds (Penicillin, Botrytis).
For best results, it is important to know how to choose, handle, and store produce.
Produce with high metabolism will spoil faster: mushrooms, berries, apricots, figs, avocados, and papayas.
Lethargic produce such as apples, pears, kiwi, cabbage, and carrots are good keepers.
Moldy fruit or vegetables should be discarded immediately, as it can lead to "an infection"and spoil the rest of the produce faster. Clean out fridge drawers and fruit bowls often to reduce the microbial population.
It's best to keep produce in restricted spaces within the fridge to slow down moisture loss, but since produce exhales carbon dioxide and water, it's important to also avoid too much condensation. (Now I get why the fridge drawers are labeled "high/low moisture!") Tip: Line containers with paper towel.
Restricted spaces will also limit access to too much oxygen, which increases metabolic activity, but not so much that the cells go into anaerobic metabolism, which generates alcohols (think fermentation) and leads to tissue damage.
It's a tricky balance because keeping produce in a closed bag will create a favorable environment of higher CO2 and low O2, but it will also trap ethylene, a plant hormone that advances ripening and accelerates aging. Permanganate inserts destroy ethylene, so inserts may extend storage life.
Cooling extends the life of produce by: 1. slowing metabolism, 2. slowing microbial growth. But beware of refrigerating fruits and vegetables that are native to warmer regions. The cold leads to cell malfunction, uncontrolled enzyme action, and tissue injury. Foods to better keep at room temperature include: melons, eggplants, squash, tomatoes, cucumbers, peppers, beans, bananas, avocados, and citrus fruits.
Freezing kills plant tissue in two ways: 1. as the water crystallizes, enzymes and other reactive molecules become concentrated and react abnormally. 2. water crystals puncture cell walls and membranes, causing a physical disruption. Flash freezing at extreme low temperatures creates smaller crystals, thus lessening the damage.
Blanching is a technique where the food is rapidly immersed in boiling water for a minute or two, then rapidly immersed in cold water, and then frozen. The boiling will inactivate enzymes, the cold water will stop the food from continuing to cook and softening the cell walls.
Sugar syrup can prevent enzymatic browning in fruit (when accompanied by ascorbic acid) and improve the texture of the frozen fruit by stiffening the cell walls when absorbed.
Frozen produce should be wrapped in air- and watertight containers to minimize freezer burn (which is caused by sublimation of water molecules).
Phew! So many Do's and Don'ts just to keep good tasting (and nutritious) fruits and vegetables.
Kitchen Chemistry
I came across a class taught at MIT called "Kitchen Chemistry." I was one class shy of being a Chemistry minor in college, and I love being in the kitchen (cooking...not so much, but baking, I love it!), so the class immediately caught my attention. To make it more fun, I asked some friends if they would join me in this exploration. For the next 14 weeks, this blog is going to be about food and the chemistry behind good cooking/baking. I will be posting notes from the book by Harold McGee, On Food and Cooking: The Science and Lore of the Kitchen.
Reason for being
The Avid Catechumen is meant to be a blog of interest. I am partial to dedicated blogs for their resourcefulness, but too many topics pique my curiosity to be the writer of one. Here the reader will find notes on subjects I'm currently exploring. From kitchen lore, to the rise of Zombie theory, to photography and other vices, join me in this learning venture to replace knowledge long forgotten.
Subscribe to:
Posts (Atom)