Baking soda, also known as sodium bicarbonate (NaHCO3), is the most common alkaline component in chemical leavenings. When mixed with an acid (H+), it reacts in the following way:
Fig 1:
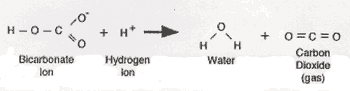
If the dough or batter contains acids, only baking soda is necessary as a chemical leavening. Common acids in baking include: sourdough cultures, buttermilk, yogurt, brown sugar, molasses, chocolate, cocoa (not Dutch processed), fruit juices, and vinegar.
Baking powders are complete leavening systems. They contain baking soda and an acid in the form of solid crystals. Ground dry starch is added to prevent premature reactions with moisture and add bulk to the mix. The timing of gas production varies according to the type of acid crystal used. If the acid is soluble (like cream of tartar), then the reactions occur quickly. If the acid is not very soluble, the reactions take longer. The acid remains in crystal form until the cooking temperature is high enough to dissolve it and promote the reaction with sodium bicarbonate.
"Double acting" baking powders inflate an initial set of gas bubbles upon mixing into the batter, and then a second set during the baking process. Baking powder for commercial purposes contains slow-release acids so that the leavening power is not lost during storage.
Chemical leaveners affect taste and color. Improper measuring and mixing can leave behind unreacted acids and bases that alter flavor. In slightly alkaline conditions, color changes occur as a result from enhanced browning reactions. Observable examples are chocolate turning red or blueberries turning green.
No comments:
Post a Comment