One of the draws of learning about what we eat is coming to understand the origin of foodstuff. I had no idea that gelatin came from animal sources. It made me realize how hard it is to adopt a vegan lifestyle. I truly respect people that do because it takes an enormous amount of effort and awareness to know where products come from and even more discipline to not be a consumer.
That said, I am once again blown away at the ingenuity of the human species. It is remarkable the stuff that people eat and how resourcefulness (i.e. using every bit of an animal/plant) has transformed food. The natural world provides us with so many delicious fruits and vegetables, it is interesting that our palates have craved for more and more diverse ways of consuming them.
On a side note, as I read about pectin and gel-formation, I could not help to reference in my brain that scene from Little Women when Meg is distressed and at her worst presentation when her husband comes home:
John: "My dearest girl, what is the matter?"
Meg: "Oh, John, I am so tired and hot and cross and worried! I've been at it till I'm all worn out. Do come and help me or I shall die!"
John: "What worries you dear? Has anything dreadful happened?"
Meg: "Yes,"
John: "Tell me quick, then. Don't cry. I can bear anything better than that. Out with it, love."
Meg: "The...The jelly won't jell and I don't know what to do!"
(text adapted from Little Women by Louisa M. Alcott, 1869.)
It made me laugh. Poor Meg did not know about pectin, sugar, and acid...
Showing posts with label Lesson7. Show all posts
Showing posts with label Lesson7. Show all posts
Monday, April 30, 2012
Berry Jam
As noted on Kitchen Chemistry by Dr. Patti Christie
Ingredients:
Method:
Strawberry or other Berry Jam
From Certo package insert
Ingredients:
- 4 cups crushed fruit (this is about 2 quarts of fresh fruit or 2.5 pounds of frozen, thawed fruit)
- 7 cup white sugar
- 1 pouch of liquid Certo
Method:
- Start the dishwasher with the jam jars. The best jars are the Mason Jars with the lid, glass jar and screw bands. To achieve the best seal, it is best to put the jam into a hot glass jar, so time the dishwasher accordingly.
- Prepare fruit. For berries, crush the fruit with a potato masher to the desired chunkiness.
- Measure the exact amount of prepared fruit into a 6 or 8 quart saucepan. It is best to use a pot in which the fruit does not go above about one quarter of the depth to prevent the jam from boiling over
- Stir sugar into fruit
- Bring mixture to full rolling boil ( a boil that does not stop bubbling when stirred) on high heat stirring constantly
- Stir in Certo quickly. Return to full rolling boil and boil exactly 1 minute stirring constantly. Remove from heat. Skim off any foam with a spoon.
- Using a 2 cup measuring cup, ladle quickly into prepared jars, filling to within 1/8 inch of tops. Wipe jar rims and threads. Cover with flat lids, then screw bands tightly.
- Invert jars for 5 minutes, then turn upright.
- After jars cool, check seals by pressing middle of lid with finger. If lid springs back, lid is not sealed and refrigeration is necessary.
- Let stand at room temperature 24 hours. Store unopened jams in a cool dark place up to 1 year. Refrigerate opened jams up to 3 weeks.
- Yield is about 8 cups of jam
Sunday, April 29, 2012
Tips in making a fruit preserve
Cook the fruit to extract the pectin. This preliminary cooking should be brief and as gentle as possible since heat and acid eventually break pectin chains. If a clear jelly is desired, the cooked fruit is strained to remove solid particles and cell debris.
Add sugar and supplemental pectin if needed. Bring the mixture to a rapid boil to concentrate the ingredients. Continue to boil until the mix reaches a temperature of 217-221 degrees F/103 - 105 degrees C at sea level. If not at sea level, adjust the temperature by lowering 2 degrees F/1 degree C per every 1000 ft/305 m elevation. At the noted temperature, the sugar concentration reaches around 65%.
Use a wide pot with a large surface area for evaporation and more gentle cooking.
Add acid in the late stages of cooking to avoid breaking the pectin molecules.
Test the readiness of the mix by placing a drop on a cold spoon and see whether it gels.
Pour into sterilized jars. The mix sets as it cools below 180 degrees F/80 degrees C. It sets most rapidly at warm room temperature (around 86 degrees F/ 30 degrees C), and continous to get firmer with time.
Failure of the mix to set can be attributed to inadequate amounts of good-quality pectin, prolonged cooking that damages the pectin, or inadequate amounts of acid. To remedy, addition of commercial liquid pectin and cream of tartar or lemon juice, and a brief reboiling should work. Too much acid causes weeping of fluid from an overfirm gel.
Uncooked and unsweeted preserves can be made by using concentrated pectin. "Freezer" jams are made by loading crushed fruit with concentrated pectin and sugar and allowed to set for a day. They are preserved in the refrigerator or freezer. Low-calorie and low-sugar jams are made by adding a modified pectin that uses cross-linking calcium to gel instead of sugar. The sugar is replaced by artificial sweeteners.
Add sugar and supplemental pectin if needed. Bring the mixture to a rapid boil to concentrate the ingredients. Continue to boil until the mix reaches a temperature of 217-221 degrees F/103 - 105 degrees C at sea level. If not at sea level, adjust the temperature by lowering 2 degrees F/1 degree C per every 1000 ft/305 m elevation. At the noted temperature, the sugar concentration reaches around 65%.
Use a wide pot with a large surface area for evaporation and more gentle cooking.
Add acid in the late stages of cooking to avoid breaking the pectin molecules.
Test the readiness of the mix by placing a drop on a cold spoon and see whether it gels.
Pour into sterilized jars. The mix sets as it cools below 180 degrees F/80 degrees C. It sets most rapidly at warm room temperature (around 86 degrees F/ 30 degrees C), and continous to get firmer with time.
Failure of the mix to set can be attributed to inadequate amounts of good-quality pectin, prolonged cooking that damages the pectin, or inadequate amounts of acid. To remedy, addition of commercial liquid pectin and cream of tartar or lemon juice, and a brief reboiling should work. Too much acid causes weeping of fluid from an overfirm gel.
Uncooked and unsweeted preserves can be made by using concentrated pectin. "Freezer" jams are made by loading crushed fruit with concentrated pectin and sugar and allowed to set for a day. They are preserved in the refrigerator or freezer. Low-calorie and low-sugar jams are made by adding a modified pectin that uses cross-linking calcium to gel instead of sugar. The sugar is replaced by artificial sweeteners.
High and low pectin fruit
Preserves can be made from all kinds of fruit, though some fruits are low in pectin and have to be mixed with either fruits high in pectin or commercial pectin to get a good gel.
Fruits high in pectin include: quince, apples, citrus fruits, cranberries, currants, gooseberries, plums, and grapes.
Fruits low in pectin include: apricots, most berries, cherries, peaches, pears, and pineapple.
Fruits high in pectin include: quince, apples, citrus fruits, cranberries, currants, gooseberries, plums, and grapes.
Fruits low in pectin include: apricots, most berries, cherries, peaches, pears, and pineapple.
Pectin gels
Pectin is a polysaccharide naturally contained in the cell walls of plants. Coupled with sugar and acid, pectin is what transforms cooked fruits into jams and jellies.
Brief history
The Ancient Greeks discovered that fruit cooked with honey developed a texture unlike any of its counterparts. By the 7th century there were several recipes for making delicate jellies made by boiling the juice of a quince with honey (quince is especially rich in pectin). The introduction of the sugar cane from Asia transformed jelly production. Unlike honey, sugar did not have excess moisture that needed to be evaporated. The Arab world used sugar to make fruit preserves through the Middle Ages, and took them to Europe in the 13th century. Still, sugar jams and jellies did not become common until after the 19th century, when sugar became cheaply available.
Transforming a jelly
Pectin, sugar, and acid are needed to make a fruit preserve, jam, or jelly. When fruit is cut up and heated near the boil, the pectin chains come off of the cell walls and dissolve into the released cell fluids and added water. Pectin molecules have the ability to bond with each other and form a meshwork that gives the jam a gel-like texture. However, pectin cannot accomplish this alone. When in solution, pectin molecules obtain a negative electrical charge that repels them from each other and they are too dilute to form a continuous network. To form the gel, sugar is added to absorb water molecules away from pectin, leaving the long chains exposed for bonding. In addition, boiling concentrates the pectin molecules as excessive water evaporates. Finally, acid provides the needed H+ that neutralize the negative electrical charge of pectin and allows the chains to come in close-enough proximity to bond. The ideal conditions for pectin gelation are: a pectin concentration of 0.5 - 1.0%; a sugar concentration of 60 -65%; and a pH between 2.8 - 3.5, about the acidity of orange juice.
Brief history
The Ancient Greeks discovered that fruit cooked with honey developed a texture unlike any of its counterparts. By the 7th century there were several recipes for making delicate jellies made by boiling the juice of a quince with honey (quince is especially rich in pectin). The introduction of the sugar cane from Asia transformed jelly production. Unlike honey, sugar did not have excess moisture that needed to be evaporated. The Arab world used sugar to make fruit preserves through the Middle Ages, and took them to Europe in the 13th century. Still, sugar jams and jellies did not become common until after the 19th century, when sugar became cheaply available.
Transforming a jelly
Pectin, sugar, and acid are needed to make a fruit preserve, jam, or jelly. When fruit is cut up and heated near the boil, the pectin chains come off of the cell walls and dissolve into the released cell fluids and added water. Pectin molecules have the ability to bond with each other and form a meshwork that gives the jam a gel-like texture. However, pectin cannot accomplish this alone. When in solution, pectin molecules obtain a negative electrical charge that repels them from each other and they are too dilute to form a continuous network. To form the gel, sugar is added to absorb water molecules away from pectin, leaving the long chains exposed for bonding. In addition, boiling concentrates the pectin molecules as excessive water evaporates. Finally, acid provides the needed H+ that neutralize the negative electrical charge of pectin and allows the chains to come in close-enough proximity to bond. The ideal conditions for pectin gelation are: a pectin concentration of 0.5 - 1.0%; a sugar concentration of 60 -65%; and a pH between 2.8 - 3.5, about the acidity of orange juice.
Saturday, April 28, 2012
Types of starch
Grain starches have some common characteristics:
- granules are medium sized
- contain significant amounts of lipids and protein
- have increased structureal stability and thus require higher temperatures to gelate
- have distinct "cereal" flavor
- contain a high proportion of moderately long amylose, so they thicken and congeal quickly
- Wheat flour is only 75% starch, which makes it a less efficient thickener than cornstarch or potatoe starch. It adds a distinct wheat flavor to the sauce. Common rule of thumb is to add 1.5 times as much flour as starch.
- Cornstarch is practically pure starch. It is an efficient thickener, but it absorbs odors and develops flavors during processing.
- Rice starch have the smallest granule size and produce a fine texture. It is seldom available in Western markets.
- larger granules that retain more water molecules
- cook faster
- release starch at lower temperatures
- contain less amylose, but the amylose chains are four times longer than cereal starches
- readily gelate
- do not require precooking to improve flavor
- Potato starch has very large granules and very long amylose chains. Stringiness and initial graininess in the sauces are notable. However, the granules are fragile and fragment easily. It is unusual in that it contains a large number of phosphate groups that carry a weak electric charge and cause the chains to repel each other. This repulsion keeps starch chains evenly dispersed in a sauce and prevents them from congealing when cool.
- Tapioca is derived from the tropical plant casava (aka manioc). It does not develop any strong aromas and it is prized for its neutral flavor. It is mostly used in puddings.
- Arrowroot starch has smaller granules than potato or tapioca starches. Its gelation temperature is higher, more comparable to that of cornstarch.
Starch
Starch is made up of thousands of glucose molecules linked up together. There are two structures of starch molecules: amylose and amylopectin. Amylose molecules are long and straight; amylopectin molecules are short and branched. Of the two, amylose is a more effective thickener than amylopectin as its long chains tangle with each other more readily and slow the motion of other molecules in the surrounding fluid.
When starch is mixed into cold water, its granules only absorb a limited amount of water and sink. Nothing happens. When starch is mixed into hot water, however, the granules absorb large amounts of water and swell up. As they do so, weak regions of the granules become disrupted; stronger regions lose their organized structure and become water-containing meshworks of long molecules. In other words, the granules become individual gels. A cloudy suspension of granules becomes more translucent as individual starch molecules become less packed and no longer deflect as many rays of light.
The temperature at which starch begins to behave in this manner is called the gelation range, usually around 120-140 degrees F/50-60 degrees C.
Thickening of a sauce/dish with starch occurs as the granules become so saturated with water that they begin to leak amylose and amylopectin molecules into the surrounding liquid. The long amylose molecules form a fishnet of sorts that entraps pockets of water and blocks the movement of the swollen starch granules.
After reaching its thickest consistency, the starch-water mixture begins to thin out again. As more amylose leaks into the water, the starch granules break or otherwise become smaller. Heating close to boiling point, vigorous stirring, continued heating long after thickening, and addition of an acid accelerate the thinning process.
The cooling that follows allows amylose molecules to form stable bonds and water molecules settle in the pockets between the starch chains. As a result, the sauce/liquid gets thicker. If the temperature drops low enough, the starch particles begin to congeal. It is important to judge the consistency of a sauce/dish at serving temperatures, not at cooking temperatures. The best way to predict the final texture of a sauce is to pour a spoonful into a cool dish and sample it.
When starch is mixed into cold water, its granules only absorb a limited amount of water and sink. Nothing happens. When starch is mixed into hot water, however, the granules absorb large amounts of water and swell up. As they do so, weak regions of the granules become disrupted; stronger regions lose their organized structure and become water-containing meshworks of long molecules. In other words, the granules become individual gels. A cloudy suspension of granules becomes more translucent as individual starch molecules become less packed and no longer deflect as many rays of light.
The temperature at which starch begins to behave in this manner is called the gelation range, usually around 120-140 degrees F/50-60 degrees C.
Thickening of a sauce/dish with starch occurs as the granules become so saturated with water that they begin to leak amylose and amylopectin molecules into the surrounding liquid. The long amylose molecules form a fishnet of sorts that entraps pockets of water and blocks the movement of the swollen starch granules.
After reaching its thickest consistency, the starch-water mixture begins to thin out again. As more amylose leaks into the water, the starch granules break or otherwise become smaller. Heating close to boiling point, vigorous stirring, continued heating long after thickening, and addition of an acid accelerate the thinning process.
The cooling that follows allows amylose molecules to form stable bonds and water molecules settle in the pockets between the starch chains. As a result, the sauce/liquid gets thicker. If the temperature drops low enough, the starch particles begin to congeal. It is important to judge the consistency of a sauce/dish at serving temperatures, not at cooking temperatures. The best way to predict the final texture of a sauce is to pour a spoonful into a cool dish and sample it.
Other gelling agents
- Carrageenan is a high-molecular weight polysaccharide obtained from red algae and has long been used in China and Ireland. It is a common food additive used as a stabilizer or emulsifier.
- Alginates come from brown seaweeds. They only form gels in the presence of calcium. They are the preferred emulsifiers for ice cream and other dairy products.
- Gellan is an industrial discovery. It is a polysaccharide produced as a fermentation product of the bacteria Sphingomonas elodea. Gellan forms a gel in the presence of salts and/or acids. Gellan is extremely versatile as it forms gels in textures ranging from hard and brittle to fluid. It is used in cosmetics and air-freshners as well as a common additive in food.
From pudding to Petri Dish
In the late 19th century, Lina Hesse, the American wife of a German scientist, recalled the advise of friends who had lived in Asia and made agar jellies and puddings that stayed solid despite the summer heat. Her husband relayed the message to his boss, microbiologist Robert Koch, who then used agar to isolate the causative agent of tuberculosis, Mycobacterium tuberculosis.
Agar gels are used as an invaluable tool in the microbiology laboratory. Scientists make agars containing various nutrients that help grow and differentiate bacteria. Agar is helpful to microbiologists because
Agar gels are used as an invaluable tool in the microbiology laboratory. Scientists make agars containing various nutrients that help grow and differentiate bacteria. Agar is helpful to microbiologists because
- very few bacteria can digest the agar carbohydrates, so agar gels remain intact and bacteria colonies separate. Bacteria readily digest the proteins in gelatin and many liquify the gel.
- Agar gels remain solid at the ideal growth temperature for most bacteria, around 100 degrees F/38 degrees C. Gelatin melts at this temperature.
Agar
Agar is a mixture of several different carbohydrates and other materials extracted from red algae. Today it is manufactured primarily by boiling the seaweeds, filtering the liquid, and freeze-drying it in strands. Solid agar pieces can be consumed raw in salads; it can be used in many sauces as a thickening agent; or used to gel flavorful mixtures of fruit juices and sugar, stews, meats, and vegetables. Agar is consumed as a jellied sweet in Japan.
Agar forms gels at much lower concentrations than gelatin. Where commercial gelatin concentration is usually >3%, agar concentrations work well under 1% by weight. The jelly is somewhat opaque, as opposed to the clear gelatin, and it has a more crumbly texture. Formed agar melts at 185 degrees F/85 degrees C, therefore it does not have the "melt-in-your-mouth" quality of gelatin. Agar gels must be chewed. The higher melting point is often desirable for cool treats that do not melt in the summer heat as well as hot dishes.
Agar forms gels at much lower concentrations than gelatin. Where commercial gelatin concentration is usually >3%, agar concentrations work well under 1% by weight. The jelly is somewhat opaque, as opposed to the clear gelatin, and it has a more crumbly texture. Formed agar melts at 185 degrees F/85 degrees C, therefore it does not have the "melt-in-your-mouth" quality of gelatin. Agar gels must be chewed. The higher melting point is often desirable for cool treats that do not melt in the summer heat as well as hot dishes.
From liquid to solid
When a gelatin solution is hot, the proteins molecules are in constant, forceful movement. As the solution cools, the molecules move more gently. The proteins form regions with helical association, i.e. they coil. A meshwork of gelatin molecules begins to form as these regions bind and align themselves. Liquid becomes trapped in the interstices of the meshwork, preventing a noticeable flow. The liquid becomes a solid gel.
Friday, April 27, 2012
Gelatin
Gelatin is a pure protein derivative that contains no fat, carbohydrates, or cholesterol and is free of all preservatives. It is most widely known for its setting qualities as a thickening and emulsifying agent in culinary uses. However, gelatin is also useful in other food processing, pharmaceuticals, photography, paper production, and other fields.
Gelatin is produced from the partial hydrolysis of collagen found in mammals. It is produced from the connective tissues, bones, and skins of animals, most commonly cows and pigs. The animal tissues undergo a process which includes cleaning, roasting, treating with acid/alkali, and boiling before gelatin is ready to be extracted. Solid gelatin is separated from its liquid components and pressed into sheets. Depending on its final purpose, it is pulverized and mixed with flavorings, colorings, sweeteners, and other additives.
Here is the chemical structure of gelatin:
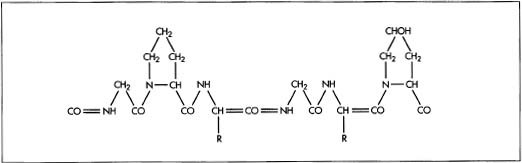
taken from: http://www.madehow.com/Volume-5/Gelatin.html
The chemical structure of gelatin gives it its versatility. It is water-soluble and forms digestible gels and films that are strong and flexible. It is transparent and has no flavor/color.
The exact history of gelatin is not documented. As it is a byproduct of animal parts, it is likely that its discovery dates back hundreds of years. Up to the 19th century, gelatin production was arduous and time consuming. In the 1840s a salesman named Charles B. Knox started packaging sheets of gelatin and selling it door to door. Peter Cooper, who had made a fortune in the manufacture of glue (a similar process to that of gelatin) received the first patent for a gelatin dessert in 1845.
In 1897, Pearl B. Wait, a cough medicine manufacturer, developed a fruit-flavored gelatin. His wife named his product Jell-O. Wait sold the rights to the process to the Genesee Food Company, for $450. In 1902, after an aggressive advertising campaign in Ladies Home Journal magazine generated enormous interest. Today, 400 million packages of Jello-O are produced each year. Over a million packages are purchased or eaten each day.
Gelatin has also had a long presence in other fields. There is documentation of the use of gelatin in paper making as early as the 14th century. In the 1870s, gelatin became a substitute for wet collodion in photography. It was used to coat dry photographic plates, marking the beginning of modern photographic methods. Today, most shells of pharmaceutical capsules are made of gelatin. Glues, paper, cosmetics, soft drinks, and many other things also contain gelatin.
Jams and Jellies
This week is another quick and easy subject. The topics include:
Gelatin jellies
Carbohydrate jellies
Thickeners
Sugar preserves
Hope you join me!
Gelatin jellies
Carbohydrate jellies
Thickeners
Sugar preserves
Hope you join me!
Subscribe to:
Posts (Atom)